Search results for leukemia
People
Not Found
Tweets including leukemia
The latest "Teaching STEM - Education News"! https://t.co/ZdpkfoIHSk Thanks to @FENews @D_Aarons @STEMHUB_SE #stemed# #leukemia#
0
0
0
0
0
Earlier this year, @UCL spinout company Autolus Therapeutics announced that the U.S. Food and Drug Administration (FDA) had granted marketing approval for AUCATZYL® (obecabtagene autoleucel, obe-cel), for the treatment of adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (r/r B-ALL).
UCLB has supported Autolus in protecting and licensing the obe-cel technology, enabling it to attract further capital to fund its development.
More here: https://t.co/ZXqNNcT9e4
#BioPharm# #TechTransfer# #Spinouts#
Show more

0
0
0
0
0
The clones have STRACK: Tracing responses to leukemic mutations https://t.co/UyOYewN3Ea


0
0
0
0
0
Mechano-oncogenic cytoskeletal remodeling drives leukemic transformation with mitochondrial vesicle-mediated STING activation https://t.co/OqySGoYTZj https://t.co/DawwfQTOpk

0
0
0
29
5
Pre-existing stem cell heterogeneity dictates clonal responses to the acquisition of leukemic driver mutations https://t.co/r1s6iSK0DM https://t.co/hAdsfsvxCD
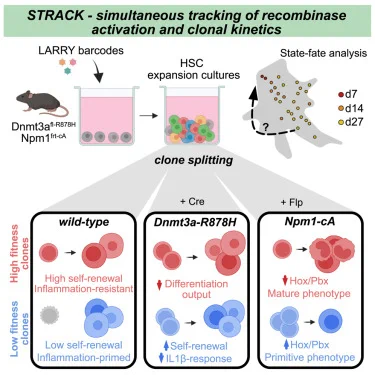
0
0
0
57
9
The @FinancialTimes has run a piece highlighting UCLB spinout Autolus Therapeutics’ journey to produce therapies for cancer side effects 👏
The news story focuses on the work taking place at Autolus’ Stevenage facility, which is manufacturing the latest generation of an innovative, personalised cancer treatment: a new Car-T cell therapy to treat the blood cancer acute lymphoblastic leukaemia.
📖 Read more here: https://t.co/3IBqRtuZsL
Show more
0
0
0
2
0


